Acidic and Basic strength (Application of I Effect)
Acidic and Basic strength
Acid = which can donate H+ and
accept lone pair.
Basic =
which can accept H+ and donate lone pair.
Note: *Negative charge on more EN-Atom is more stable.
=> HO-
is more stable than H2C-
-> 8
protons -> 6 protons
-> more
attraction
So, more stable
Note: *Positive charge on less EN-Atom is more stable.
=> H3C+
is more stable than HO+
-> 6
protons -> 8
protons
-> more repulsion
So, less stable
[A] Acidic Strength
1. CH4
< NH3 < H2O < HF
-> H3C- H2N- HO- F- {Conjugate base}
-> -ve
charge on less EN
-> -ve charge on more EN
-> Less
stable H3C- ->
more stable F-
-> Less
forming of H3C- -> more
forming of F-
-> Less
breaking of CH4 -> more
breaking of HF
-> Less H+
from CH4
-> more H+ from HF
Note: *Acidic strength
stability of conjugate base
i.e. More stable conjugate base more acidic character of corresponding acid.
2. H3C-CH3 < H2C=CH2 < HC≡CH
-> H3C-C-H2 H2C=C-H HC≡C- {Conjugate
base}
-> -ve on
less EN (SP3 C) -> -ve on
more EN (SP C)
-> less
stable conjugate base
-> more stable conjugate base
3. H3C-CH2-OH > (CH3)2-CH-OH >
(CH3)3-C-OH
-> H3C-CH2-O- (CH3)2-CH-O- (CH3)3-C-O- {Conjugate base}
-> Less
+I effect of ethyl group -> more
+I effect of butyl group
-> Less
–ve charge on O-atom -> more
–ve charge on O-atom
-> more
stable conjugate base -> less
stable conjugate base
4. NO2-CH2-COOH >
H-CH2-COOH > CH3-CH2-COOH
-> NO2-CH2-COO- H-CH2-COO- CH3-CH2-COO- {Conjugate base}
-> More +I effect
-> More –ve charge
-> Less stable
conjugate base
So, cross pounding acid is less acidic
Example:
a. H3C-CH2-COOH
> (CH3)2-CH-COOH > (CH3)3-C-COOH
+I Effect
increases
========================================>
So, acidic
strength decreases
b. Cl3C-CH2-COOH > Cl2HC-CH-COOH > ClH2C-C-COOH
-I Effect decreases
=========================================>
So, acidic strength decreases
c. CH3-CH2-CHF-COOH >
CH3-CHF-CH2-COOH
> F-(CH2)3-COOH
Distance of Fluorine atom increases so,
-I Effect decreases =========================================>
That’s why acidic strength decreases
d. COOH-COOH >
COOH-CH2-COOH
> COOH-CH2-CH2-COOH
{Oxalic acid} {Malonic acid} {Succinic acid}
==========================================>
+I Effect increases so, acidic
strength decreases
e. CH3-(CH2)2-COOH <
CH2=CH-CH3-COOH
< CH≡C-CH2-COOH
==========================================>
-I Effect increases ==========================================>
S-Character increases
So, EN increases
So, -ve charge decreases
That’s why stability increases
Question:
Which of the following
have maximum Pka
Options:
a. CH3-CF-COOH
b. F-CH2-CH2-COOH
c. CH3-CHBr-COOH
d. CH2Br-CH2-COOH
Answer: (d)
Reason:
Minimum –I Effect
Minimum acidic strength
Minimum Ka
Maximum Pka
Question:
Arrange the
followings in correct order of acidic strength.
1.
a. ClCH2COOH b. BrCH2COOH c.
CH3COOH d. FCH2COOH
Order: d > a > b > c
2.
a.
R-CO-OH b. H-OH c. R-OH d. CH≡CH
R-CO-O- HO- R-O- CH≡C-
-> More
stable due to resonance
-> -ve on less EN
And –I Effect of (R-CO-) group
so less stable
Order: a > b > c > d
[B] Basic Strength
1. CH3-CH2-NH2 > H-CH2-NH2 >
NO2-CH2-NH2
-> +I
Effect -> No Effect -> -I Effect
-> More
electron density -> less electron density
-> More
tendency
-> less tendency
to donate lone pair
to donate lone pair
-> More
basic
-> less basic
2. H3C-CH2-OH
< (CH3)2-CH-OH
< (CH3)3-C-OH
-> Less
+I Effect
-> More
+I Effect
-> Less electron
density
-> more electron density
-> Less
tendency
-> more tendency
to donate lone pair to donate lone pair
-> Less
basic
-> more basic
3. CH3-CH2-NH2
> CH3-NH2 > CH3-OH
-> More
+I Effect -> less +I
Effect
-> More
electron density -> less electron
density
-> More
tendency -> less
tendency
to donate lone pair to donate lone pair
-> More
basic ->
less basic
-> Lone pair on more EN atom
->
So more stable
-> Less tendency
to donate lone pair
-> Least basic
*Note: Amines are more basic than Alcohol.
4. a. CH3-NH2 b. (CH3)2-NH c. (CH3)3-N
-> More +I Effect
-> More electron density
-> More tendency to donate lone pair
but H+ less acceptable lone pair
due to Steric hindrance.
Order of
basic strength:
(CH3)2-NH > (CH3)-NH2 > (CH3)3-N
{Secondary
amine} {Primary amine} {Tertiary amine}
*Basic
strength depends on:-
-> Inductive effect
-> Steric hindrance
-> Hydration energy
So,
Dimethylamine is more basic than Trimethylamine in aqueous solution.
Reason:-
a. Less
steric hindrance of Dimethylamine than Trimethylamine.
b. More
hydration energy of Dimethylamine than Trimethylamine.
Question:
Hydration
Question:-
1. (CH3)2NH
is more basic than (CH3)3N in aqueous solution why?
Answer:-
-> Less
steric hindrance in (CH3)2NH than (CH3)3N.
-> More
hydration energy of (CH3)2NH than (CH3)3N.
2. Explain
following order of Kb in aqueous solution.
(Et)2NH >
(Et)3N > (Et)NH3 {Et = Ethyl group (-CH2CH3)}
Answer:-
-> (Et)2NH
is more basic than (Et)3N because of less steric hindrance and
more hydration energy of (Et)2NH.
-> (Et)3N
is more basic than (Et)NH3 because of more +I Effect in (Et)3N.
Order of
basic strength in gaseous state {Aprotic solution}.
a. R3-N >
R2-NH > R-NH3 >
NH3 {R =
Alkyl group}
b. H3C- >
H2N-
> HO- >
F-
-> -ve on
less EN atom ->
-ve on more EN atom
-> less
stable
-> more stable
-> So, less donate lone pair and less basic
c. CH3-C-H2 > CH2=C-H > CH≡C-
-> -ve on
less EN (SP3 C) -> -ve on more EN (SP
C)
-> less
stable
-> more stable
-> easily
lone pair donation ->
not easily lone pair donation
-> more
basic
-> less basic
d. F- > Cl- > Br- > I-
-> -ve charge on large sized atom
-> more stable –ve charge or lone pair
-> less tendency to donate lone pair
->
so, less basic
Order of
acidic strength:-
HF
< CCl <
HBr < HI
e. CH3-N-H > CH3-O-
> CH3-S-
-> -ve
charge on less EN -> -ve charge on
larger size
-> less
stable
-> more stable
-> more
tendency to donate lone pair
-> less tendency to donate lone pair
-> more
basic -> less basic
Question:-
Arrange in
correct order of stability.
So, Order:-
a >
b > c
> d
Thanks for reading..:)

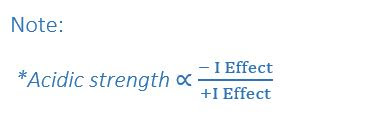



0 comments:
Post a Comment